Wechselwirkung aquatischer Kolloide mit Mineraloberflächen
- Betreuung:Prof. Dr. D. Bosbach
- Bearbeitung:
Dr. Andre Filby
Motivation
Interaction of colloids with mineral surfaces
Aquatic colloids are ubiquitous in natural groundwater. Depending on surface charge, geochemical conditions and structure, colloids can adsorb radionuclides onto their surfaces or incorporate them. Thus, radionuclides released from a nuclear waste disposal site can be transported colloid-mediated over considerable distances. On the other hand, colloids can also be retained by interaction with mineral surfaces. The interaction of colloids as radionuclide carriers with natural mineral surfaces is complex and only insufficiently understood. A complete understanding of the processes involved is complicated, for example, by the chemical heterogeneity and roughness of the mineral surfaces, discrete surface charges and by surface contaminations.
The present work describes experimental results on sorption experiments with carboxylated polystyrene colloids and natural rock and mineral surfaces. In these experiments, the pH is varied over a wide range while the ionic strength of the colloidal suspension is maintained constant. Sorption of the colloids on the mineral surfaces is made visible by means of fluorescence microscopy and the observed fluorescence is assigned to specific mineral phases. Using different analytical techniques, such as Scanning Electron Microscopy (SEM), energy-dispersive X-Ray spectroscopy (EDX) those mineral phases are to be identified on which a predominant colloid sorption takes place.
Experimental
For the sorption experiments carboxylated polystyrene colloids (Postnova Analytics; Landsberg/Lech, Germany) with a diameter of 25 nm are used. The colloids have a polydispersity index of < 0.1 and a density of COOH groups of 120 nmol/mg. The natural rock samples (Grimsel granodiorite) used are from a water-bearing fracture in the underground Grimsel rock laboratory. For the experiments these rock samples were cut into small cubes and contacted with the colloid suspension. In the experiments rhodamine-doped colloids with an excitation wavelength of 552 nm and an emission wavelength of 580 nm were used. After contacting the rock samples for 15 minutes with the colloid suspension, the rock samples were rinsed with MQ-water and dried in an oven. After this procedure, fluorescence can be observed by means of fluorescence microscopy. The pH was varied from 2 – 10 and the ionic strength was kept constant at 0.01 M with NaCl. The colloid concentration was set to 0.05 g/l. For ease of handling, the initial experiments were carried out with polished rock surfaces. Experiments with natural (unpolished) surfaces were also carried out; these surfaces were treated with chloroform, methanol and MQ-water to remove organic impurities which also may contribute to the fluorescence signal.
Results
Only weak fluorescence intensities were observed from natural surfaces under neutral and alkaline pH conditions (6 – 10; I = 0.01 M NaCl). Colloids are mainly found at mineral edges. At pH < 4 colloids adsorb preferentially to Apatite, Titanite, Illite and Feldspars. Sorption always takes place on the natural (unpolished) surfaces. High pH should prevent the sorption of colloids, since at high pH there are repulsive conditions for the relevant minerals (Quartz, Feldspars, Mica, Biotite, Illite, Apatite). Especially in regions with high surface roughness, considerable sorption is observed regardless of pH. Since on polished surfaces there are only very few mineral edges available, there is far less adsorption observed.
Further experiments have been carried out with some of the individual minerals occurring in the Grimsel granodiorite, such as Quartz, Muscovite, Biotite, Orthoclase, Albite, Apatite and Titanite. Their sorption behaviour has been observed at pH = 2 - 10 and an ionic strength of 0.01 M NaCl. Colloids do not sorb onto quartz, while adsorption onto all other minerals could be identified at pH values from 2 - 6. At pH 10 sorption was only observed on Titanite. Additionally, similar experiments have been carried out with addition of 10-5 M Eu3+ at pH values from 2 – 6 and 10-6 M Eu3+ at pH values of 8 and 10. These experiments showed a strong influence of Eu3+, especially at higher pHs. In contrast to the experiments without Eu3+, sorption took place on every mineral at pH = 10, with the exception of Quartz.
Fig. 1 shows a Muscovite sample viewed with the fluorescence microscope after carrying out an experiment at pH = 4 and I = 0,01 M (NaCl) without addition of Eu3+. Sorption, indicated by fluorescence, is only visible on the positively charged crystal steps, wheras no sorption is detectable on the permanently negative charged (and thus repulsive) basal surfaces, since the carboxylic groups on the colloid surfaces are partly deprotonated under these conditions.
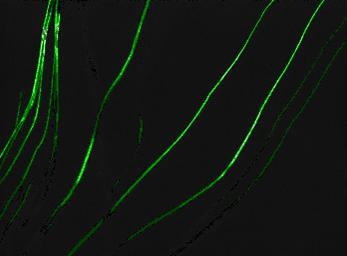 |
Figure 1: Fluorescence-optical image of Muscovite; pH = 4, ccolloids = 0,05 g/l, I = 0,01 M (NaCl). Sorption is only viewed on the crystal steps. |
Fig. 2 shows a Muscovite sample under same conditions as Fig. 1, but with addition of 10-5 M Eu3+. Obviously, under these conditions, sorption of colloids takes place not only on the mineral edges, but also on the negative charged surface. A plausible binding mechanism between negatively charged colloids and negatively charged micaceous mineral surfaces would be polyvalent cation bridging, in this case by way of Eu3+.
| Figure 2: Fluorescence-optical image of Muscovite; pH = 4, ccolloids = 0,05 g/l, I = 0,01 M (NaCl), [Eu3+] = 10-5 M. Sorption is detected over the the whole mineral surface. | 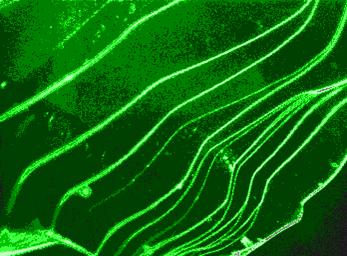 |
Preliminary results
Adsorption of carboxylated polystyrene colloids increases with decreasing pH, both on natural (rough) and polished surfaces. Although on the polished surfaces, no adsorption is observed at higher pH-values (> 6), on the natural surface adsorption is observed over the whole pH range, despite the repulsive conditions expected at higher pH-values
Experiments with single minerals naturally occurring in Grimsel Granodiorite show a strong influence of Eu3+, especially at high (for the Grimsel system relevant) pHs.
Future sorption experiments will be carried out with variations in ionic strength and background electrolyte (e.g. CaCl2).
Recently, initial experiments using atomic force microscopy (AFM) were performed in order to obtain force-distance curves between Muscovite and a carboxylated polystyrene “colloidal probe” (diameter 1 µm). It is planned to obtain such curves with several relevant mineral phases identified in the sorption experiments in order to distinguish the forces responsible for the differing modes of interaction of colloids with the various mineral phases.