Remediation of Chromium (VI)-Contaminated Groundwater Using Green Rust and Other Layered Double Hydroxides
- Betreuung:
Prof. Dr. T. Neumann
- Bearbeitung:
 |
 |
 |
 |
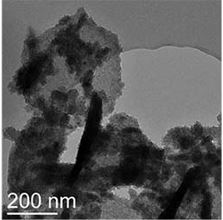
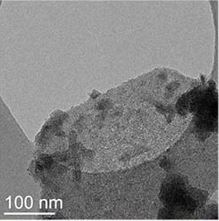
TEM and SEM images of samples from reactions C1 ([C]= 67.6 mg/kg, a and b) and C6 ([Cr]= 1.6 mg/kg, c and d) after 7 days.
Hexavalent chromium is a toxic, carcinogenic metal commonly found in soils impacted by contamination from metal plating factories, wood treatment plants and tanneries. Previously, efforts to remediation groundwater contamination by hexavalent chromium using in situ methods have aimed at reducing Cr (VI) to Cr (III), which is less soluble and less toxic than its hexavalent equivalent. Existing methods capable of causing this transformation include Fe2+/dithionite, sulfide-bearing reagents and zero valent iron, but reductive transformation of Cr (VI) is often not permanent due to redox processes native to contaminated soils. Biogenic Mn (IV) oxides such as δ-MnO2 have been shown to oxidize aqueous Cr3+ and can oxidize significant portions of solid Cr (III) in soils over time, particularly if the Cr (III)-bearing phases are sufficiently soluble. Therefore, any remediation technique applied to reductively transform Cr (VI) in soils should aim to minimize the solubility of the Cr (III)-bearing solid produced. Green rust sulfate, a layered Fe (II, III) oxyhydroxide, is a heterogeneous reactive particle capable of converting hexavalent chromium to an insoluble Cr (III)-bearing iron oxide if it reacts at the preferred site in the green rust particle’s interlayer. My experiments focus on how isomorphic substitution of metallic ions and addition of sorbents to block reactive sites at the particle surface affect the reactivity and reaction products of hexavalent chromium reduction by green rust.